Note
INOSOLVE constantly invests in its own equipment and in the training of its qualifiers. The team of qualifiers thinks holistically and accompanies you from the planning phase up to the Rountine operation!
Qualification & Validation
INOSOLVE owns extensive state of the art equipment from ELLAB, Climate, Ametek, Rotronic, which is constantly invested in. The qualification and validation team has many years of experience in this area. The team attaches great importance to accurate and meaningful documentation.
Your advantages:
- Due to the large equipment park, high availability (parallel use possible) and flexible application possibilities. We can measure up to 100 measuring points simultaneously.
- If you do not have your own qualification system on the subject of cleanroom temperature and sterilization, you can access our quality-assured SOPs (INOSOLVE documents have also been checked by customers through EMA inspections and thus confirm the high quality of the documentation)
- Our team has many years of experience especially in the cleanroom and therefore also the knowledge about the correct behavior.
Our services:
Preparation of documents (e.g.: specification documents, master plans, risk analyses)
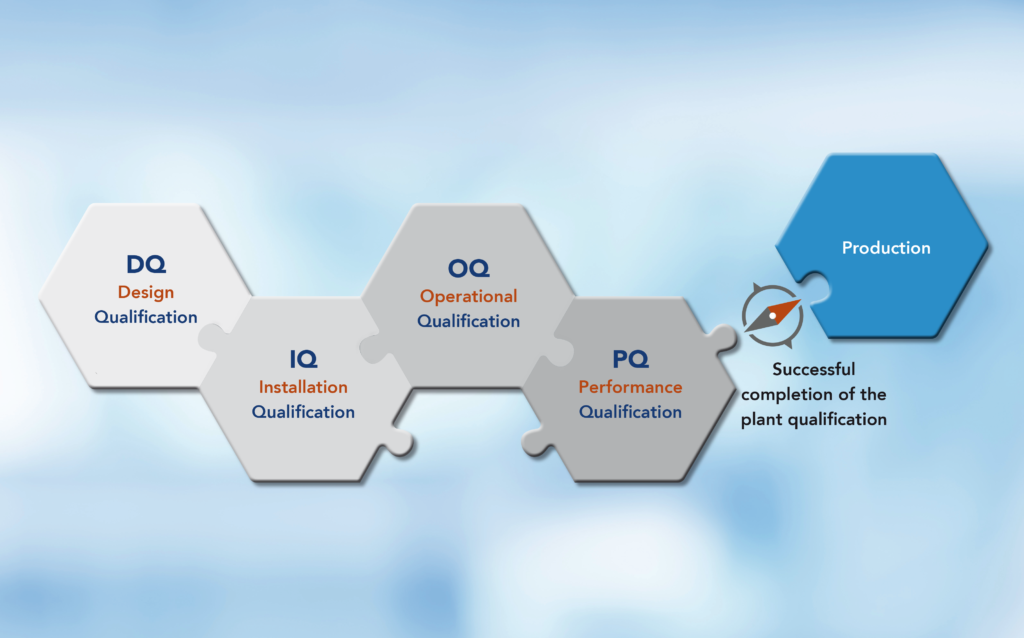
- Preparation of DQ/IQ/OQ/PQ protocols, plans, etc.
- Cleanroom qualification
- Cleaning validation
- Sterilization validation
- Process validation
- Transport validation
- Validation of computer systems
- Provision of measurement equipment
- Measuring equipment management at the customer
- On-site calibrations of the common physical
- Parameters (temperature, humidity, pressure, CO2 concentration, additional parameters possible on request)
- Advice on the purchase of equipment and facilities.
Read more
Our services:
Preparation of documents (e.g.: specification documents, master plans, risk analyses)
- Preparation of DQ/IQ/OQ/PQ protocols, plans, etc. Clean room qualification
Cleaning validation
Sterilization validation - Process validation
Transport validation
Validation of computer systems
Provision of measurement equipment
Measuring equipment management at the customer
On-site calibrations of the common physical - Parameters (temperature, humidity, pressure, CO2 concentration, additional parameters possible on request)
- Advice on the purchase of equipment and facilities.